
Notch Therapeutics: Solving the Big Issues In Cell Therapy

Wrote my company's blog post on why we invested in Notch Therapeutics. You can find the original here
This week, Notch Therapeutics announced an oversubscribed Series A financing of $85M and we are excited to be part of the syndicate that is building this leading cell therapy company. Amplitude’s investment strategy is to create, build and grow highly innovative Precision Medicine companies. We are excited about Canada emerging as a leader in the targeted and cell therapy space and Notch is another great example of a world-leading Canadian company in this space.
The founding scientific team formed the company around innovations from Peter Zandstra and Juan Carlos Zúñiga-Pflücker’s pioneering work at the University of Toronto, CCRM and Sunnybrook Research Institute. We were first introduced to the technology and team via our engagement with Creative Destruction Lab in Vancouver and mentored them during their tenure. We’ve closely followed their progress through their growth into the Notch we know today. The company is now emerging as a world leader in the allogeneic cell therapy space with a truly cross-Canada story. Working closely with the Centre for the Commercialization of Regenerative Medicine (CCRM) and having key team members in Vancouver, Toronto and expanding into Seattle, the company has growing access to talent, resources, and infrastructure.
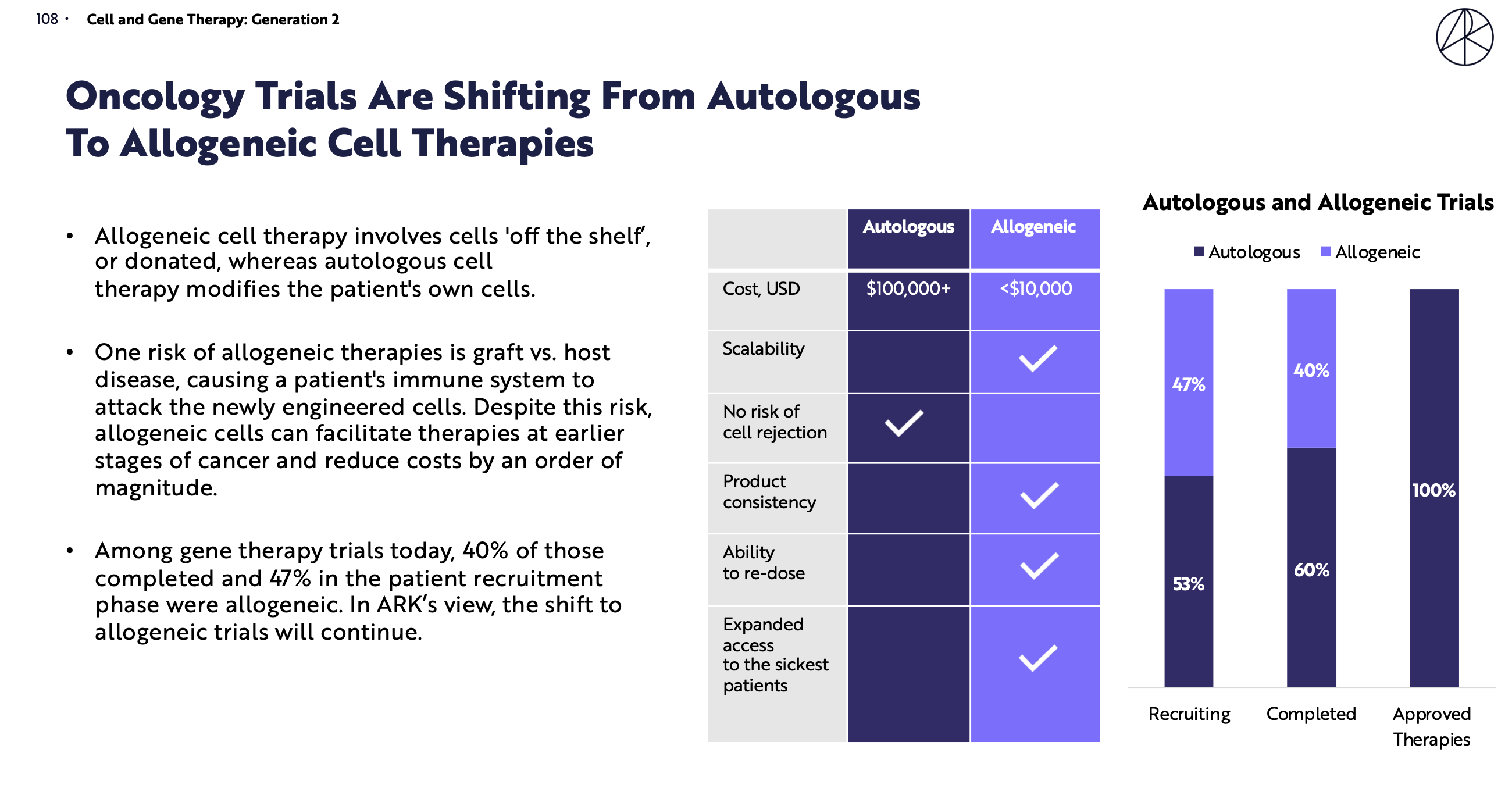
Cell therapies have emerged in recent years as a powerful tool for treating cancer patients when other treatments have failed. The concept of harnessing a patient’s own immune system to fight cancer is not new, but the strategy of modifying their own cells (autologous therapy) and donor cells (allogeneic therapy) has resulted in impressive recoveries in a significant number of patients.
The use of autologous (patient-derived) second-generation CAR-T cells has resulted in frequent complete responses in patients with hematological cancers that were previously considered incurable. This led to the approval of three agents, tisagenlecleucel (Kymriah/Novartis), axicabtagene ciloleucel (Yescarata, which was acquired by Gilead from Kite Pharma for $11.9 billion dollars) and lisocabtagene maraleucel (Juno/Bristol-Myers-Squibb).
However, since their approval, these CAR-T therapies have not been as widely used as they could be due to several issues. These issues include complex logistics, delayed manufacturing timelines, limited patient eligibility, high costs, and low-quality T cells. The emergence of severe adverse events like cytokine storm and the inability to penetrate solid tumors have also been difficult challenges and limit the uptake of autologous cell therapy.
Instead of using the patient’s own cells for treatment, several companies, including Notch, are developing ‘off-the-shelf’ allogeneic cell therapy, which aims to solve many of the issues that have plagued autologous cell therapy. An off-the-shelf product simplifies logistics, allows companies to manufacture cells that can be stored and used on demand, lowers the cost of treatment for hospitals and patients, allows more patients to be eligible for treatment, and potentially gives higher quality T cells that are more homogenous in nature.
Allogeneic cell therapies are still very early in their development. Allogene Therapeutics, one of the most advanced companies in this area, presented the first clinical trial readout of an allogeneic product at ASH in December 2020, with some preliminary data from another phase I trial presented at ASCO in 2020. Other issues remain to be solved including the potential for graft vs. host disease, where cells sourced from a healthy donor may attack a patient’s immune system, as well as infiltration into solid tumors, toxicities, suboptimal persistence in the patient and moderately high costs.
In the cell therapy field, the process is the product, and it is clear that allogeneic outperforms autologous on a number of aspects of the process. We are not alone in this belief as evidenced by the stellar syndicate that Notch was able to attract, as well as key opinion leaders and investors in the space who understand that cell therapies are moving from autologous to allogeneic. ARK Invest, a leading investment company focused on disruptive technologies and innovative companies, recently published their ‘Big Ideas 2021’ report and cited the shift of cell and gene therapies from autologous to allogeneic as one of their big ideas, potentially increasing the total addressable market by $70 billion.
Companies are using a variety of different approaches to unlock the full potential of this treatment, but we believe that Notch has a unique and sustainable advantage over many of them. Using their proprietary engineered thymic niche (ETN) technology platform, Notch hopes to develop homogenous and universally compatible stem-cell-derived cell therapies. To date, the company has assembled a world-class scientific and management team and built a fully integrated platform for generating and editing immune cells from pluripotent stem cells. The company has demonstrated the ability for large-quantity production of T cells and others from any source of stem cells using their ETN platform. Their technology has the potential to generate immunotherapies with decreased variability, increased potency, and engineered improvements.
Amplitude is incredibly bullish on the potential and performance of life sciences companies in Canada, and our confidence in the sector is only amplified by the recent success of companies like Repare Therapeutics and Zymeworks. Along with supporting incredible science, the Canadian government understands the critical need for domestic bio-manufacturing going forward and has committed hundreds of millions of dollars across the country in order to bolster Canada’s manufacturing capabilities. This will only help to further the ability of domestic companies to compete globally.
Notch is another great example of a company at the bleeding edge of their field with global ambitions and access to local resources such as the leading stem cell hub in Vancouver and CCRM in Toronto. We know that with an exceptional team, a proprietary platform that can generate off-the-shelf cell therapies, and a shift in the market from autologous to allogeneic, Notch has positioned itself as a leader in the space for years to come.
We are thrilled this company is in our portfolio. With the continuous innovation happening in Canada, it feels like we’re only at the beginning.
-Amplitude Team